Metals need to be identified before welding to pick the correct electrode and method.
At a minimum, an entry-level welder should have the ability to:
- Distinguish between the common metals
- Recognize common shop materials (channel, tubing, elbows, flanges, etc.) and structural members (I-beams, trusses, girders, etc.)
- Understand the basic metalworking processes (especially heat treatment) and vocabulary
Welding school graduates should also be able to:
- Identify unmarked metals using a spark test, magnet, or other means
- Interpret the meaning of stock classification numbers on labels and supply bins
The following pages will walk you through the various tests, physical, mechanical, and visual properties used to determine metals’ origin.
Most of the metals and alloys described in this site section can be welded by one or more major welding processes (Stick, TIG, MIG, Oxyfuel).
This section describes the characteristics of metals and their alloys, particularly their significance in welding operations.
All metals fall within two categories, ferrous or nonferrous.
- Ferrous metals – are metals that contain iron.
Ferrous metals appear in the form of cast iron, carbon steel, and tool steel. The various alloys of iron, after undergoing certain processes, are pig iron, gray cast iron, white iron, white cast iron, malleable cast iron, wrought iron, alloy steel, and carbon steel. All these types of iron are mixtures of iron and carbon, manganese, sulfur, silicon, and phosphorous. Other elements are also present, but in amounts that do not appreciably affect the characteristics of the metal. - Nonferrous metals – are those which do not contain iron.
Aluminum, copper, magnesium, and titanium alloys are among those metals which belong to this group.

Physical Properties of Metals
Many of the physical properties of metals determine if and how they can be welded and how they will perform in service.
Physical properties comprise several metal ID methods shown in table 7-1 a&b below.
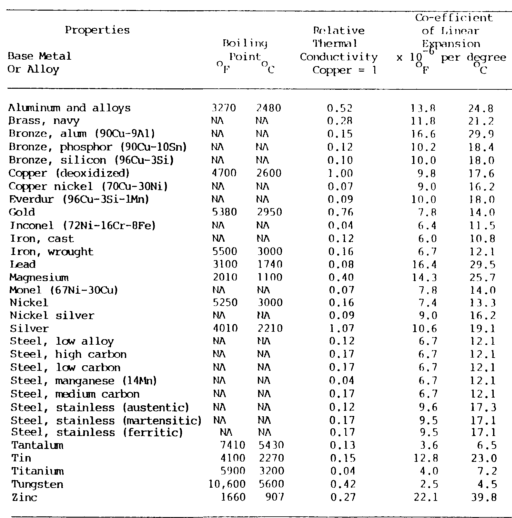
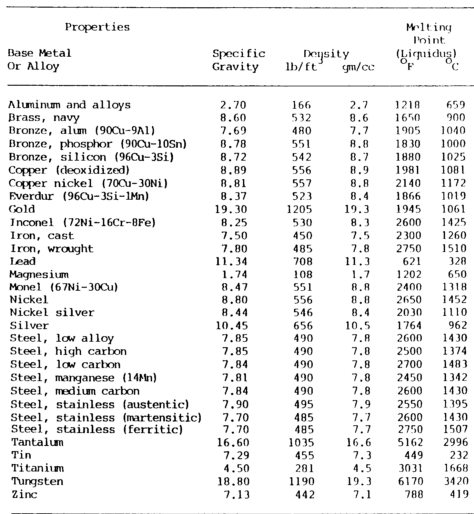
Metal Color
Color relates to the quality of light reflected from the metal.
Mass or Density
Mass or density relates to mass with respect to volume.
Commonly known as specific gravity, this property is the ratio of the mass of a given volume of the metal to the mass of the same volume of water at a specified temperature, usually 39°F (4°C).
For example, the ratio of the weight of one cubic foot of water to one cubic foot of cast iron is the specific gravity of cast iron. This property is measured by grams per cubic millimeter or centimeter in the metric system.
Melting Point
Knowing the melting point of a metal is important when welding.
A metal’s fusibility is related to its melting point, the temperature at which it changes from a solid to a molten state.
Pure substances have a sharp melting point and pass from a solid-state to a liquid without changing temperature.
However, during this process, there is an absorption of heat during the melting and liberation of heat during freezing.
The absorption or release of thermal energy when a substance changes state is called its latent heat.
Mercury is the only common metal that is in its molten state at normal room temperature. Metals having low melting temperatures can be welded with lower temperature heat sources. The soldering and brazing processes utilize low-temperature metals to join metals having higher melting temperatures.
Related read: Welding Metals
Boiling Point
The boiling point is also an important factor in welding.
The boiling point is when the metal changes from the liquid state to the vapor state. Some metals, when exposed to the heat of an arc, will vaporize.
Conductivity
Thermal and electrical conductivity relate to the metal’s ability to conduct or transfer heat and electricity.
Thermal conductivity
Thermal conductivity is the ability of a metal to transmit heat throughout its mass, which is of vital importance in welding since one metal may transmit heat from the welding area much more quickly than another.
The thermal conductivity of a metal indicates the need for preheating and the size of the heat source required.
Thermal conductivity is usually related to copper. Copper has the highest thermal conductivity of the common metals, exceeded only by silver.
Aluminum has approximately half the thermal conductivity of copper, and steels have about one-tenth the conductivity of copper.
Thermal conductivity is measured in calories per square centimeter per second per degree Celsius.
Electrical conductivity
Electrical conductivity is the capacity of metal to conduct an electric current.
A measure of electrical conductivity is provided by the ability of a metal to conduct the passage of electrical current.
Its opposite is resistivity, measured in micro-ohms per cubic centimeter at a standardized temperature, usually 20°C. Electrical conductivity is usually considered as a percentage and is related to copper or silver.
Temperature bears an important part in this property. As the temperature of a metal increases, its conductivity decreases.
This property is fundamental to resistance welding and electrical circuits.
Coefficient of Linear Thermal Expansion
With few exceptions, solids expand when they are heated and contract when they are cooled.
The coefficient of linear thermal expansion measures the linear increase per unit length based on the change in temperature of the metal.
Expansion is the increase in the dimension of a metal caused by heat. The expansion of metal in a longitudinal direction is known as linear expansion.
The linear expansion coefficient is expressed as the linear expansion per unit length for one degree of temperature increase.
When metals increase in size, they increase not only in length but also in breadth and thickness. This is called volumetric expansion.
The coefficient of linear and volumetric expansion varies over a wide range for different metals.
Aluminum has the greatest expansion coefficient, expanding almost twice as much as steel for the same temperature change.
This is important for welding for warpage, warpage control, and fixturing and for welding together dissimilar metals.
Corrosion Resistance
Corrosion resistance is the resistance to eating or wearing away by air, moisture, or other agents.
The mechanical properties of metals determine the range of usefulness of the metal and establish the service needed.
Visually Identifying Common Metals
Carbon Steel

This is an inexpensive metal used for commercial buildings, bridges, pipelines, and other types of construction.
After the iron is extracted from the ore, its carbon content is manipulated to produce different steel grades.
Steel with higher amounts of carbon is harder, which is why cutting tools have a lot of it. On the downside, harder can mean brittle.
Most structural steel contains lower amounts of carbon to remain ductile. The flexibility allows the metal to respond to various loads, impacts, temperatures, and seismic disturbances without cracking.
Low-carbon steel can also take a lot of heat in welding without any serious consequences.
High carbon steels are more sensitive to heat. Usually, they require a pre or post-heat treatment to relieve any stresses or changes in grain size and lattice structure that may develop whenever the metal is welded.
Steel is a dense, relatively heavy material that easily rusts, so the surface must be painted, galvanized, cleaned often, encased in concrete, or protected in some other way.
Freshly ground carbon steel looks shiny and metallic; otherwise, it has a dull, dark (but still metallic) color.
On a grinder, steel produces lots of sparks, as a rule, the greater the spark bursts, the higher the carbon content of the steel.
There are four basic grades of steel, and not easy to tell apart by appearance alone:
- Mild Steel – used in pipe and steel framing 0.05% to 0.3% carbon
- Medium Carbon Steel – forging and vehicle parts 0.3% to 0.6 % carbon
- High-Carbon Steel – springs and wire 0.6% to 1% carbon
- Tool Steel – cutting and drilling tools 1% – 2% carbon
If the iron content is higher than 2%, the metal is classified as wrought or cast iron. At this point, it becomes much harder to weld with any success.
Stainless Steel

This is carbon steel with ten percent or more chromium added to prevent rust and other forms of corrosion.
For this reason, stainless steel is the preferred metal for containers that hold food, liquids, and chemicals.
Restaurant kitchens are packed with stainless steel counters, pots, coffee urns, and, of course, silverware.
You can recognize it by its grainy look and silvery glean.
Other alloying elements commonly included are nickel and molybdenum.
There are three categories of stainless steel based on the lattice structure of the metal when it’s manufactured: martensitic, ferritic, and austenitic.
These classifications are impossible to distinguish visually. However, there are still ways to tell them apart. The austenitic type is non-magnetic.
- Austenitic – The most common and extensive range of stainless steel and non-magnetic. The type most frequently used is 18-8 (18% Chromium and 8% Nickel). It’s highly corrosion resistant and thus used for food and chemical equipment, architectural and structural applications.
- Ferritic – Usually made from a straight chromium alloy with no additions. It’s magnetic and not heat-treatable. Mostly used for automotive trim and cooking utensils.
- Martensitic – Used to make fasteners, pump shafts, and turbine blades. Magnetic.
Here are two other less common stainless steel classifications:
- Precipitation Hardening – A chromium-nickel blend that’s made stronger by special heat treatment. The stainless steels may be referred to by chromium-nickel percentages, such as 13-8, 15-5, or 17-7. Used to make valves, gears, and petrochemical equipment.
- Duplex – A blend of three alloys: chromium, nickel, and molybdenum. It’s more resistant to stress corrosion cracking than austenitic, yet tougher than ferritic alloys. Used for pipelines.
Read: Welding Stainless Steel: A Complete Guide
Galvanized Steel
Galvanized steel is carbon steel that has been submerged in molten zinc. The zinc provides a protective coating that resists rust and corrosion.
As in the familiar Cyclone fencing, galvanized steel appears a solid, bright gray color.
Zinc is considered a toxic substance, so you shouldn’t weld on galvanized steel until the zinc coating has been ground away from around the weld joint.
And you shouldn’t grind zinc without wearing a respirator or sturdy N-95 dust mask.
Aluminum

This metal is extracted from bauxite ore and then subjected to lengthy processing, which involves electrolysis.
Because of its complicated recipe, aluminum only came into vogue during modern times.
Besides soda cans and aluminum foil, it’s used to construct airplanes, automotive components, and other things that require the toughness of steel but not the weight.
It’s not ferrous, so it doesn’t rust and always remains shiny. Aluminum doesn’t produce any sparks on a grinder.
Related Read: How Do You Join Aluminum to Steel?
Chromoly
A well-known commodity around custom auto shops, this metal is a class of alloyed steel.
Chromoly stands for chromium-molybdenum, but nickel and other elements may be included in the recipe.
The metal is used for gears, piston pins, connectors, frames, and crankshafts.
It can be subjected to a heat treatment known as case-hardening to make it more durable. Chromoly produces lots of sparks on a grinder.
Titanium
This lightweight material is wildly expensive, so you’re not likely to find it casually lying around a shop.
Yet, it pays for itself with excellent mechanical properties and the highest strength-to-weight ratio among the metals.
Its high corrosion resistance includes saltwater, chlorine, and even acids.
Welded titanium pipe and equipment (like pressure vessels and tanks) are used by the petrochemical industry.
The metal has long been incorporated in aerospace applications, and more recently, in high-end motorcycles, bicycle frames, tennis rackets, and golf clubs.
Because they’re non-toxic, titanium plates, hips, and screws are also planted nowadays inside human beings with good results.
Cast Iron

Best known as the metal of pancake griddles, skillets, and soup pots, this metal is chock-full of carbon. It cannot be formed because high carbon content means brittleness which also makes cast iron difficult to weld.
Instead, the metal has to be poured into molds, which is why it’s called “cast” iron.
You see it everywhere in metal shops since cast iron forms the base of many stationary shop tools.
The metal is heavy-duty and resists deformation, making it the perfect choice for long-lasting, level surfaces for work tables and saws.
Related: Metal Casting
Wrought Iron
In ancient times, iron ores were smelted in charcoal fires.
Since charcoal is pure carbon, the process adds lots of carbon, producing wrought iron.
In a blacksmith’s forge, air or oxygen is used to stoke a fire that may reduce carbon from 4 or 5 percent to about 2 percent.
Now the iron is ductile enough to form (or forge) with hammers, punches, and other tools.
Wrought iron is used for ornamental ironwork, door latches and hardware, barnyard tools, and other blacksmithing implements.
Copper
Onee the go-to metal for plumbing, the role of copper in the world, has diminished somewhat since the advent of PVC and copper ore’s rising price tag.
Even pennies are made of zinc now, with a copper coating.
However, this noble metal is still used in electric wire and transformers since it conducts electric current better than anything else and resists corrosion.
Bronze
This is a combination of copper and a second metal, usually tin, which produces a strong, tough substance that resists corrosion better than iron.
In antiquity, bronze first gained its widespread reputation in cutting sculptures, knives and swords. (Ancients initially combined copper with arsenic to make the bronze, not tin, which meant an early trip to the grave for many bronzesmiths.)
Before the advent of stainless steel, bronze was the primary metal for boat and ship fittings, and it’s still used to construct ship propellers.
TIG welders use silicon-bronze brazing rod on sheet metal panels since it generates less distortion and warping.
Brass

More than decorative because of its shiny, gold-like look, brass is used in plumbing and electrical connectors because it resists static electricity and sparks.
Due to exceptional acoustic properties, you may also be familiar with brass on musical instruments (like tubas and horns).
The metal is softer and lighter than bronze and combines copper with zinc (rather than tin).
In a welding shop, most of the fittings on compressed gas cylinders and torches — such as the flashback arrestors in the photo above — are made of brass.
Lead
Contrary to popular belief, pencils are not made out of lead but graphite.
Lead is an extremely dense metal used in medical settings as a shield from X-rays and other forms of radiation.
The metal is gray and has a low melting point, which makes it ideal for soldering.
However, it’s no longer used as a plumbing pipe or to solder canned foods because it’s toxic enough to cause nerve disorders.
